Note
Go to the end to download the full example code.
Flow Cytometry Simulation: Full System Example#
This tutorial demonstrates a complete flow cytometry simulation using the FlowCyPy library. It models fluidics, optics, signal processing, and classification of multiple particle populations.
Steps Covered:#
Configure simulation parameters and noise models
Define laser source, flow cell geometry, and fluidics
Add synthetic particle populations
Set up detectors, amplifier, and digitizer
Simulate analog and digital signal acquisition
Apply triggering and peak detection
Classify particle events based on peak features
Step 0: Global Settings and Imports#
import numpy as np
from TypedUnit import ureg
from FlowCyPy import SimulationSettings
SimulationSettings.include_noises = False
SimulationSettings.include_shot_noise = True
SimulationSettings.include_dark_current_noise = True
SimulationSettings.include_source_noise = True
SimulationSettings.include_amplifier_noise = True
SimulationSettings.assume_perfect_hydrodynamic_focusing = True
np.random.seed(3)
Step 1: Define Flow Cell and Fluidics#
from FlowCyPy.flow_cell import FlowCell
from FlowCyPy.fluidics import Fluidics, ScattererCollection, distribution, population
from FlowCyPy.sampling_method import GammaModel, ExplicitModel
flow_cell = FlowCell(
sample_volume_flow=80 * ureg.microliter / ureg.minute,
sheath_volume_flow=1 * ureg.milliliter / ureg.minute,
width=200 * ureg.micrometer,
height=100 * ureg.micrometer,
)
scatterer_collection = ScattererCollection()
medium_refractive_index = distribution.Delta(1.33 * ureg.RIU)
population_0 = population.Sphere(
name="Pop 0",
medium_refractive_index=medium_refractive_index,
particle_count=5e17 * ureg.particle / ureg.milliliter,
diameter=distribution.RosinRammler(50 * ureg.nanometer, spread=30),
refractive_index=distribution.Normal(
1.44 * ureg.RIU, standard_deviation=0.002 * ureg.RIU
),
sampling_method=GammaModel(mc_samples=10_000),
)
population_1 = population.Sphere(
name="Pop 1",
particle_count=5e9 * ureg.particle / ureg.milliliter,
medium_refractive_index=medium_refractive_index,
diameter=distribution.RosinRammler(150 * ureg.nanometer, spread=30),
refractive_index=distribution.Normal(
1.44 * ureg.RIU, standard_deviation=0.002 * ureg.RIU
),
)
scatterer_collection.add_population(population_0, population_1)
scatterer_collection.dilute(factor=80)
fluidics = Fluidics(scatterer_collection=scatterer_collection, flow_cell=flow_cell)
Step 2: Define Optical Subsystem#
from FlowCyPy.opto_electronics import (
Detector,
OptoElectronics,
TransimpedanceAmplifier,
source,
)
source = source.GaussianBeam(
numerical_aperture=0.1 * ureg.AU,
wavelength=405 * ureg.nanometer,
optical_power=200 * ureg.milliwatt,
RIN=-180,
)
detectors = [
Detector(
name="forward",
phi_angle=0 * ureg.degree,
numerical_aperture=0.3 * ureg.AU,
responsivity=1 * ureg.ampere / ureg.watt,
),
Detector(
name="side",
phi_angle=90 * ureg.degree,
numerical_aperture=0.3 * ureg.AU,
responsivity=1 * ureg.ampere / ureg.watt,
),
Detector(
name="det 2",
phi_angle=30 * ureg.degree,
numerical_aperture=0.3 * ureg.AU,
responsivity=1 * ureg.ampere / ureg.watt,
),
]
amplifier = TransimpedanceAmplifier(
gain=10 * ureg.volt / ureg.ampere,
bandwidth=10 * ureg.megahertz,
voltage_noise_density=0.1 * ureg.nanovolt / ureg.sqrt_hertz,
current_noise_density=0.2 * ureg.femtoampere / ureg.sqrt_hertz,
)
opto_electronics = OptoElectronics(
detectors=detectors, source=source, amplifier=amplifier
)
Step 3: Signal Processing Configuration#
from FlowCyPy.signal_processing import (
Digitizer,
SignalProcessing,
circuits,
peak_locator,
triggering_system,
)
digitizer = Digitizer(
bit_depth="14bit", saturation_levels="auto", sampling_rate=60 * ureg.megahertz
)
analog_processing = [
circuits.BaselineRestorator(window_size=10 * ureg.microsecond),
circuits.BesselLowPass(cutoff=2 * ureg.megahertz, order=4, gain=2),
]
triggering = triggering_system.DynamicWindow(
trigger_detector_name="forward",
threshold=10 * ureg.microvolt,
pre_buffer=20,
post_buffer=20,
max_triggers=-1,
)
peak_algo = peak_locator.GlobalPeakLocator(compute_width=False)
signal_processing = SignalProcessing(
digitizer=digitizer,
analog_processing=analog_processing,
triggering_system=triggering,
peak_algorithm=peak_algo,
)
Step 4: Run Simulation#
from FlowCyPy import FlowCytometer
cytometer = FlowCytometer(
opto_electronics=opto_electronics,
fluidics=fluidics,
signal_processing=signal_processing,
background_power=0.001 * ureg.milliwatt,
)
run_record = cytometer.run(run_time=1 * ureg.millisecond)
Step 5: Plot Events and Raw Analog Signals#
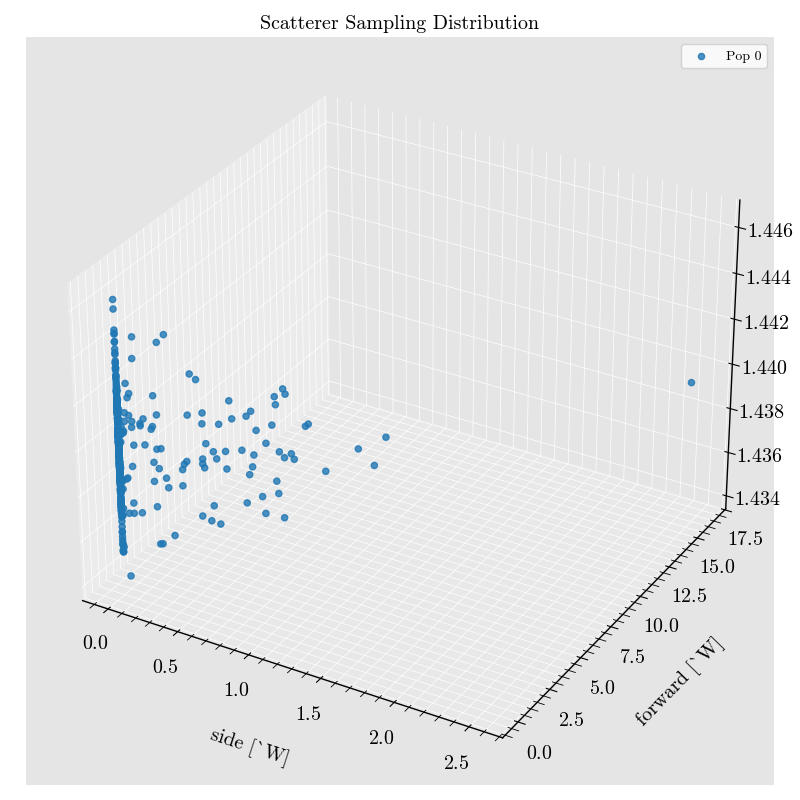
_ = run_record.event_collection.plot(x="side")
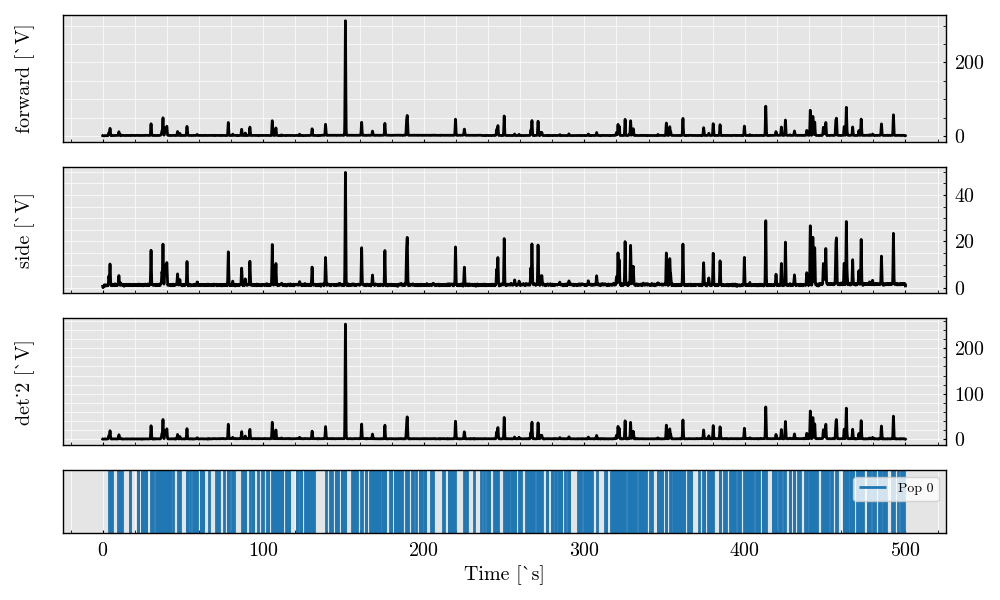
Plot raw analog signals#
_ = run_record.plot_analog(figure_size=(12, 8))
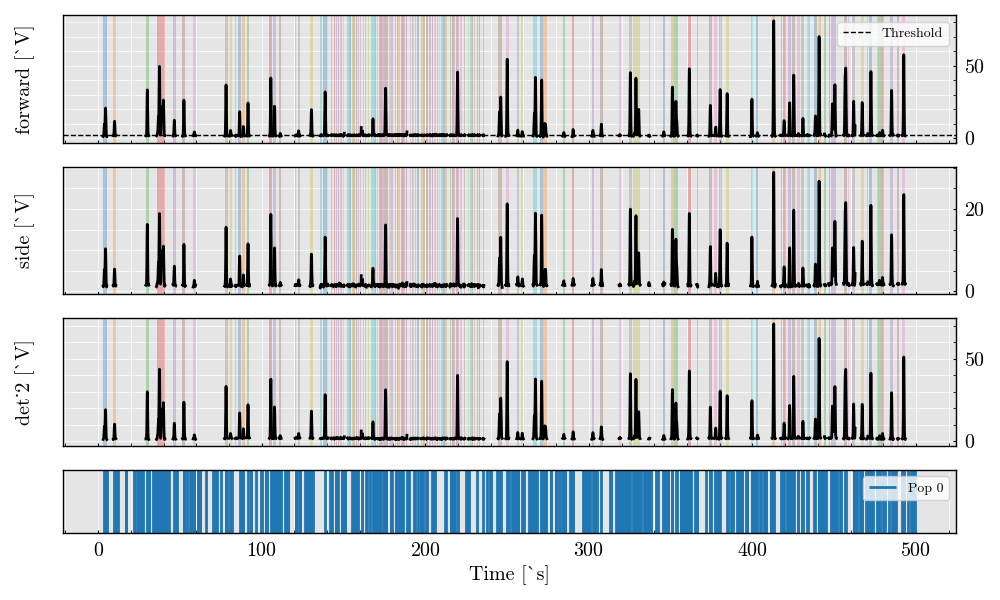
Step 6: Plot Triggered Analog Segments#
_ = run_record.plot_digital(figure_size=(12, 8))
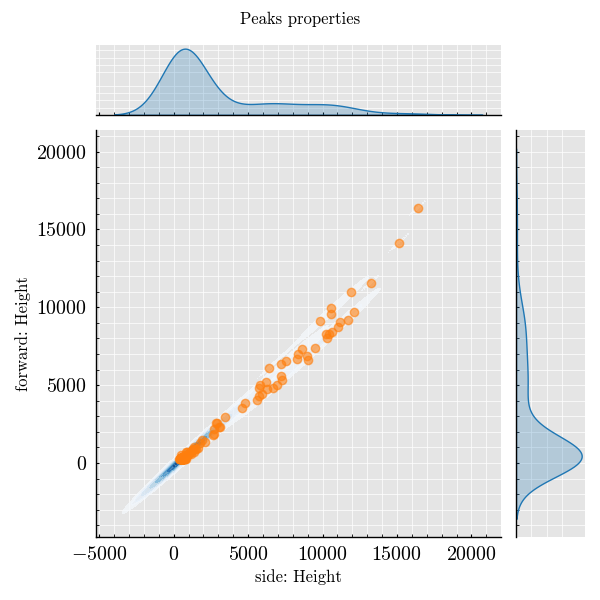
Step 7: Plot Peak Features#
_ = run_record.peaks.plot(x=("forward", "Height"))
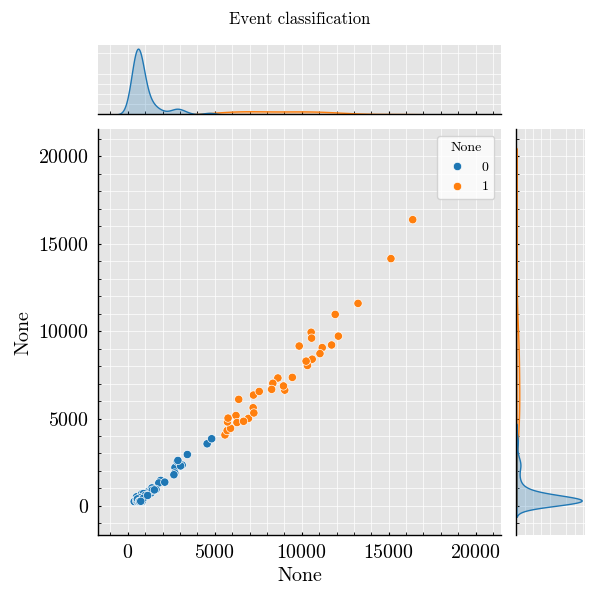
Step 8: Classify Events from Peak Features#
from FlowCyPy.classifier import KmeansClassifier
classifier = KmeansClassifier(number_of_clusters=2)
classified = classifier.run(
dataframe=run_record.peaks.unstack("Detector"),
features=["Height"],
detectors=["side", "forward"],
)
_ = classified.plot(x=("side", "Height"), y=("forward", "Height"))
Total running time of the script: (0 minutes 11.016 seconds)